Cannabis Vape Cartridge Contaminants: A Growing Concern
Vitamin E Acetate (VEA)
In 2023, Michigan’s cannabis market reached a significant milestone, surpassing $3 billion in sales. Vape cartridges accounted for approximately 19% of these sales, translating to a substantial $581.4 million. The popularity of vape cartridges stems from their ease of use and convenience, making them a preferred method for consuming cannabinoids. However, their rise to prominence has not been without challenges1.
In August 2019, the first cases of E-cigarette/Vaping use Associated Lung Injury (EVALI) emerged, marking a turning point in the industry2–6. The cause? Vitamin E Acetate (VEA), a substance often added by black market providers to make vape concentrates appear more premium and produce impressive plumes of white vapor7. Although VEA is FDA-approved for oral consumption, it has not been approved for inhalation. When heated and inhaled, VEA can coat the alveoli in the lungs, leading to severe respiratory issues, including labored breathing, asthma, lipoid pneumonia, and, in some cases, death6.
Medium Chain Triglycerides (MCT Oil)
Another contaminant of concern in vape cartridges is Medium Chain Triglycerides (MCT Oil), commonly derived from refined coconut oil and widely available as a dietary supplement. MCT oil consists of 3 fatty acids, carbon chains ranging from 6 to 12 in length, attached to a glycerol as shown in the figure below.
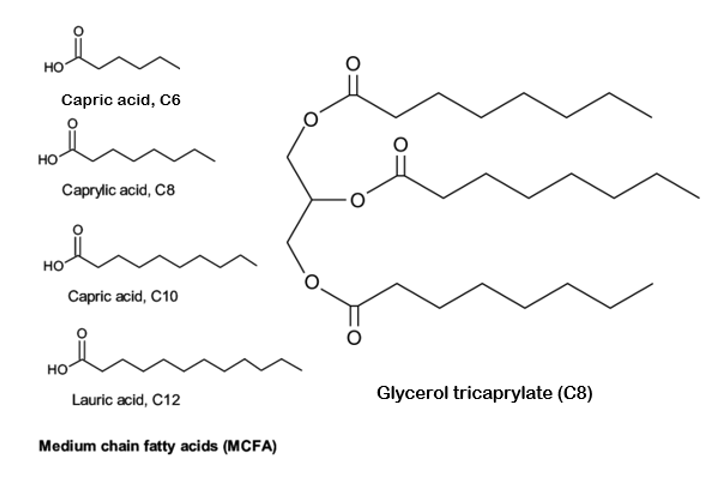
Like VEA, MCT oil has been found in vape cartridges, although clinical data on its effects is limited. What we do know is alarming: when MCT oil is heated to 230°C, it produces harmful compounds such as Acetaldehyde, Acrolein, and Formaldehyde—all of which are toxic and carcinogenic to humans7. Moreover, vape cartridges adulterated with these additives result in inferior, potentially dangerous products. Starting in October 2024, Michigan’s Cannabis Regulatory Agency will require all vape cartridges to be screened for MCT oil in addition to VEA.
Chemically Converted Cannabinoids
The 2018 Farm Bill’s legalization of low-THC hemp production has led to an influx of chemically converted cannabinoid products, particularly Δ9-THC and THCA derived from CBD extracted from hemp. These conversions often occur in unregulated facilities, lacking the required licensing, product tracking, and safety compliance testing.
While properly synthesized cannabinoids can be difficult to detect analytically, the reality is that many of these conversions are performed by individuals lacking the necessary expertise, using substandard chemicals. Furthermore, the conversion processes often take place in acidic environments as timed reactions. Without proper clean-up and stabilization, these processes leave behind a host of chemical by-products detectable by advanced analytical techniques like Gas Chromatography-Mass Spectrometry (GC-MS) and Liquid Chromatography-Mass Spectrometry (LC-MS).
Why Choose Therapeutic Health Choice?
At Therapeutic Health Choice, we understand the importance of product safety in the rapidly evolving cannabis market. That’s why we’ve developed a robust analytical method using GC-MS to detect the presence of VEA, MCT oil triglycerides, and the various intermediaries found during the chemical conversion of CBD into Δ9-THC, Δ8-THC, and THCA.
Our commitment to rigorous testing ensures that you can trust the safety and quality of your vape cartridges. Don’t leave your product’s integrity to chance—contact us today to learn how we can help safeguard your business and protect your customers.
References
(1) January 16, T. L.; 2024. Michigan Caps Off $3 Billion Year in Cannabis Sales With Record-Breaking December. Cannabis Business Times. https://www.cannabisbusinesstimes.com/news/michigan-cannabis-record-sales-3-billion-2023-prices/ (accessed 2024-08-26).
(2) Werner, A. K.; Koumans, E. H.; Chatham-Stephens, K.; Salvatore, P. P.; Armatas, C.; Byers, P.; Clark, C. R.; Ghinai, I.; Holzbauer, S. M.; Navarette, K. A.; Danielson, M. L.; Ellington, S.; Moritz, E. D.; Petersen, E. E.; Kiernan, E. A.; Baldwin, G. T.; Briss, P.; Jones, C. M.; King, B. A.; Krishnasamy, V.; Rose, D. A.; Reagan-Steiner, S. Hospitalizations and Deaths Associated with EVALI. N. Engl. J. Med. 2020, 382 (17), 1589–1598. https://doi.org/10.1056/NEJMoa1915314.
(3) Winnicka, L.; Shenoy, M. A. EVALI and the Pulmonary Toxicity of Electronic Cigarettes: A Review. J. Gen. Intern. Med. 2020, 35 (7), 2130–2135. https://doi.org/10.1007/s11606-020-05813-2.
(4) The Lancet Respiratory Medicine. The EVALI Outbreak and Vaping in the COVID-19 Era. Lancet Respir. Med. 2020, 8 (9), 831. https://doi.org/10.1016/S2213-2600(20)30360-X.
(5) Kalininskiy, A.; Bach, C. T.; Nacca, N. E.; Ginsberg, G.; Marraffa, J.; Navarette, K. A.; McGraw, M. D.; Croft, D. P. E-Cigarette, or Vaping, Product Use Associated Lung Injury (EVALI): Case Series and Diagnostic Approach. Lancet Respir. Med. 2019, 7 (12), 1017–1026. https://doi.org/10.1016/S2213-2600(19)30415-1.
(6) Marrocco, A.; Singh, D.; Christiani, D. C.; Demokritou, P. E-Cigarette Vaping Associated Acute Lung Injury (EVALI): State of Science and Future Research Needs. Crit. Rev. Toxicol. 2022, 52 (3), 188–220. https://doi.org/10.1080/10408444.2022.2082918.
(7) Troutt, W. D.; DiDonato, M. D. Carbonyl Compounds Produced by Vaporizing Cannabis Oil Thinning Agents. J. Altern. Complement. Med. 2017, 23 (11), 879–884. https://doi.org/10.1089/acm.2016.0337.
(8) Jadhav, H. B.; Annapure, U. S. Triglycerides of Medium-Chain Fatty Acids: A Concise Review. J. Food Sci. Technol. 2023, 60 (8), 2143–2152. https://doi.org/10.1007/s13197-022-05499-w.
